From the February 2007 Idaho Observer:
HPV vaccines: Cancer preventative or cancer cause?
News that Texas Governor Rick Perry ® signed an executive order mandating that girls receive the human papilloma virus (HPV) vaccine to prevent cervical cancer prompted a huge wave of opposition—from sea to shining sea. The resulting outrage did compel Texan legislators to act, letting their constituents know that Perry’s order has no authority without legislative oversight. However, the announcement was probably a "trial balloon" to measure public response to an unjustified vaccine mandate. The incident illuminated many issues that are basic to the most fundamental aspects of science, politics and the rights of people to manage their own health care issues.
by Dewey R. Duffel
Introduction
Gardasil® was licensed by the FDA on June 8, 2006 as a vaccine against human papillomavirus (HPV), the virus associated with cervical* cancer. Great success in trials is claimed. We expect the reality to be much more sobering than the hype. Indeed, vaccines have been associated with an increase in cancer for 150 years. This trend has never ended as Polio vaccines and even the flu vaccine have been implicated as cancer causing. It is best to live a healthy lifestyle and keep toxic compounds, which all vaccines contain, out of your body.
Important Facts: Most women do not experience any symptoms from contact with human papillomavirus. Immunity is natural. It has been demonstrated that a healthy immune system clears the virus quickly.
Risk Factors:
- Diet – "Women with diets low in fruits and vegetables may be at increased risk for cervical cancer. Also overweight women are more likely to develop this cancer." Source: www.cancer.org
- Use of oral contraceptives.
- Smoking.
- Tampons – contain dioxin contaminated bleaching agent
- Other exposure to toxic compounds in the food, air and water.
(We expect that the vaccine itself will be a contributor to cancer).
Cost Effective?
In 2004, there were 10,500 cases of cervical cancer for which about 70 percent, 7350 cases, were associated with the four viruses targeted by Gardasil. IF the vaccine works, which is unlikely and, IF it prevents all of the 7350 targeted cases, an impossibility, and IF the 2004 rate remains constant rather than continuing the established 35 year decline, then it requires 272 girls to be vaccinated to prevent one case of cervical cancer. Estimated costs for the Gardasil three shot series range from $300 to $500. Thus each "vaccine preventable" case will cost between $81,600 and $136,000. Given that cervical cancer is virtually 100 percent preventable by lifestyle factors and readily treatable, plus the vaccine will cause other diseases that increase the estimated cost for each cancer prevented, you will appreciate why we urge you to strive for immunity through lifestyle not vaccination. In addition, the concept that vaccinating nine year old girls will prevent a disease which peaks at age 40 to 55, seems like a story which should begin, "Once upon a time, the wagon of the snake oil salesman was seen approaching ..." See our statistics section at www.vaclib.org/news/2006/gardasil.htm#stats.
Methodology?
What was the quality of the vaccine trial methodology?
Cervical cancer rates have been dropping for several years. The cervical cancer death rate declined 45 percent between the periods 1972-74 and 1992-94 and the overall incidence of the disease has decreased steadily from 14.2 per 100,000 in 1973 to 7.4 per 100,000 in 1995. Source: www.hhs.gov/asl/testify/t990316b.html
Much the same is true in Canada.
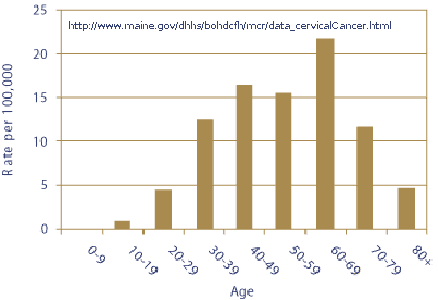
Note: In the above graph that 9-year-old girls are not shown to have cervical cancer, 10-19 year old girls are expected to have 1 cervical cancer case per year per 100,000. In the 20-29 year old women less than 5 cases are expected per 100,000 women. Merck "tested" the vaccine in 9 to 26 year old women. In 100,000 women in the 9-26 year old age group one would expect about 3-4 cervical cancer cases per year (depending upon the age distribution). In four years that would equate to 12 to 16 cases in 100,000 and if the trial contained only one-tenth as many subjects (10,000), that would translate to 1 to 2 cases. The average follow-up was 2.4 years for 20,541 subjects with an expected number of cervical cancer cases totaling one to three. Thus Merck’s trial contained too few subjects for too short a period of time of follow-up for evaluating cervical cancer incidence. In the table below you can see that only the first 2391 subjects had a 4-year follow-up.
Analysis of the follow-up
Note: The Gardasil package insert mentions, "21,464 total subjects (9- to 26-year-old girls and women and 9- to 15- year-old boys)".
Conclusions:
Known or feared adverse effects of Gardasil
An FDA review of the results of studies on the vaccine found two important concerns, according to the documents released ahead of Thursday’s meeting of the Vaccines and Related Biological Products advisory committee.
- The first is that the vaccine may lead to an increased number of cases of a cancer precursor among patients already infected by any of the four virus types at the time they receive the vaccine, and whose immune systems have not cleared the virus from their bodies.
- The second concern is that any advantage the vaccine provides in protecting against the four virus types could be offset by infection by any of the multiple other types of HPV that the vaccine does not cover, according to the FDA documents.
- FDA staff also asked that the committee examine five cases where children with birth defects were born to women who had received the vaccine around the time of conception.
Gardasil is given in three injections over six months and will cost $300-$500.
From the package insert for Gardasil:
"Description
GARDASIL is a non-infectious recombinant, quadrivalent vaccine prepared from the highly purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV types 6, 11, 16 and 18. The L1 proteins are produced by separate fermentations in recombinant Saccharomyces cerevisiae and self-assembled into VLPs. The fermentation process involves growth of S. cerevisiae on chemically-defined fermentation media which include vitamins, amino acids, mineral salts, and carbohydrates. The VLPs are released from the yeast cells by cell disruption and purified by a series of chemical and physical methods. The purified VLPs are adsorbed on preformed aluminum-containing adjuvant (amorphous aluminum hydroxyphosphate sulfate). The quadrivalent HPV VLP vaccine is a sterile liquid suspension that is prepared by combining the adsorbed VLPs of each HPV type and additional amounts of the aluminum-containing adjuvant and the final purification buffer.
"GARDASIL is a steril preparation for intramuscular administration. Each 0.5-mL dose contains approximately 20 mcg of HPV 6 L1 protein, 40 mcg of 11 L1 protein, 40 mcg of HPV 16 L1 protein, and 20 mcg of HPV 18 L1 protein.
"Each 0.5-mL dose of the vaccine contains approximately 225 mcg of aluminum (as amorphous aluminum hydroxyphosphate sulfate), 9.56 mg of sodium chloride, 0.78 mg of L-histidine, 50 mcg of polysorbate 80, 35 mcg of sodium borate, and water for injection."
Claims for effectiveness in preventing cancer are based on indirect efficacy measurements. The number of subjects, the age of the subjects (9-26) and the duration of the trials was such that no cases of cancer were recorded in either the Placebo or Gardasil groups. In other words, there is no proof that even one case of cervical cancer has been prevented to date by this vaccine.
Placebo: Table 6 of the package insert lists 3470 subjects receiving Aluminum-Containing Placebo and only 320 containing Saline Placebo. [Note: Aluminum-Containing Placebo is not a Placebo and would bias the results.]
Not recommended for pregnant women
GARDASIL is not recommended for use in pregnant women. There were 15 cases of congenital anomaly in pregnancies that occurred in subjects who received GARDASIL and 16 cases of congenital anomaly in pregnancies that occurred in subjects who received (the aluminum-containing) placebo. Further sub-analyses were conducted to evaluate pregnancies with estimated onset within 30 days, or more than 30 days from administration of a dose of GARDASIL or placebo. For pregnancies with estimated onset within 30 days of vaccination, 5 cases of congenital anomaly were observed in the group that received GARDASIL, including pyloric stenosis, congenital megacolon, congenital hydronephrosis, hip dysplasia and club foot. The other anomalies occurred in pregnancies with onset more than 30 days following vaccination. None was judged by the investigator to be vaccine related.
In clinical studies, a higher number of breast-feeding infants whose mothers received GARDASIL had acute respiratory illnesses within 30 days post-vaccination of the mother as compared to those whose mothers received placebo.
Oil-based adjuvants
There are currently two Human Papillomavirus vaccines: Gardasil by Merck Pharmaceuticals and Cervarix by GlaxoSmithKline(GSK.) Cervarix is currently undergoing review by the FDA and GSK anticipates its approval sometime this year. Unlike Gardasil that contains four HPV viruses and is recommended for women 9 to 26 years of age, Cervarix contains only two HPV viruses, types 16 and 18, and is recommended for women up to age 55.
Cervarix is formulated with GSK’s new proprietary adjuvant system, AS04 that was developed with the goal of inducing strong and sustained immune responses. "AS04 is composed of aluminum salt and monophosphoryl lipid A (MPL); MPL is an immuno-stimulant capable of directly activating key immune mechanisms, which will ultimately enhance the immune response to the antigens included in the vaccine."
It should be noted that MPL is what we would consider an "oil-based" adjuvant with properties similar to the squalene used in anthrax vaccines. For an amazing, and frightening, history of oil-based adjuvants, read Gary Matsumoto’s book, Vaccine A: The Covert Government Experiment That’s Killing Our Soldiers and Why GIs Are Only the First Victims.
According to Matsumoto, the first classic oil adjuvant, Freund’s Complete Adjuvant, was used in the 50s in animal vaccine experiments since it had the ability to trigger "an extremely powerful immune response." Dr. Jules Freund warned in 1956 that animals injected with his formulation developed terrible, incurable conditions: Allergic aspermatogenesis (cessation of sperm production), allergic encephalomyelitis (the animal version of MS), allergic neuritis (inflammation of the nerves that can lead to paralysis) and other autoimmune disorders.
Due to these debilitating effects in animals, scientists of the day never considered oil-based adjuvants for vaccines developed for human use.
We now know that by injecting a lipid or fatty acid into the body, this actually causes the body to produce antibodies to its own essential fatty acids, the building blocks of the central nervous system. On the jacket of Vaccine A, Matsumoto mentions "an Army sergeant [a recipient of six anthrax vaccines] whose cerebellum literally shrank until he could no longer walk straight or write his name legibly."
Internet source for this article - http://www.vaclib.org/news/2006/gardasil.htm
Home - Current Edition
Advertising Rate Sheet
About the Idaho Observer
Some recent articles
Some older articles
Why we're here
Subscribe
Our Writers
Corrections and Clarifications
Hari Heath
Vaccination Liberation - vaclib.org